Do you hope to find 'synthesis of acetylsalicylic acid'? Here you can find the answers.
Table of contents
- Synthesis of acetylsalicylic acid in 2021
- Synthesis of acetylsalicylic acid lab report
- Acetylsalicylic acid bond line
- Synthesis of aspirin lab report discussion pdf
- Synthesis of aspirin balanced equation
- Preparation of aspirin project pdf
- Synthesis of aspirin mechanism
- Industrial production of acetylsalicylic acid
Synthesis of acetylsalicylic acid in 2021
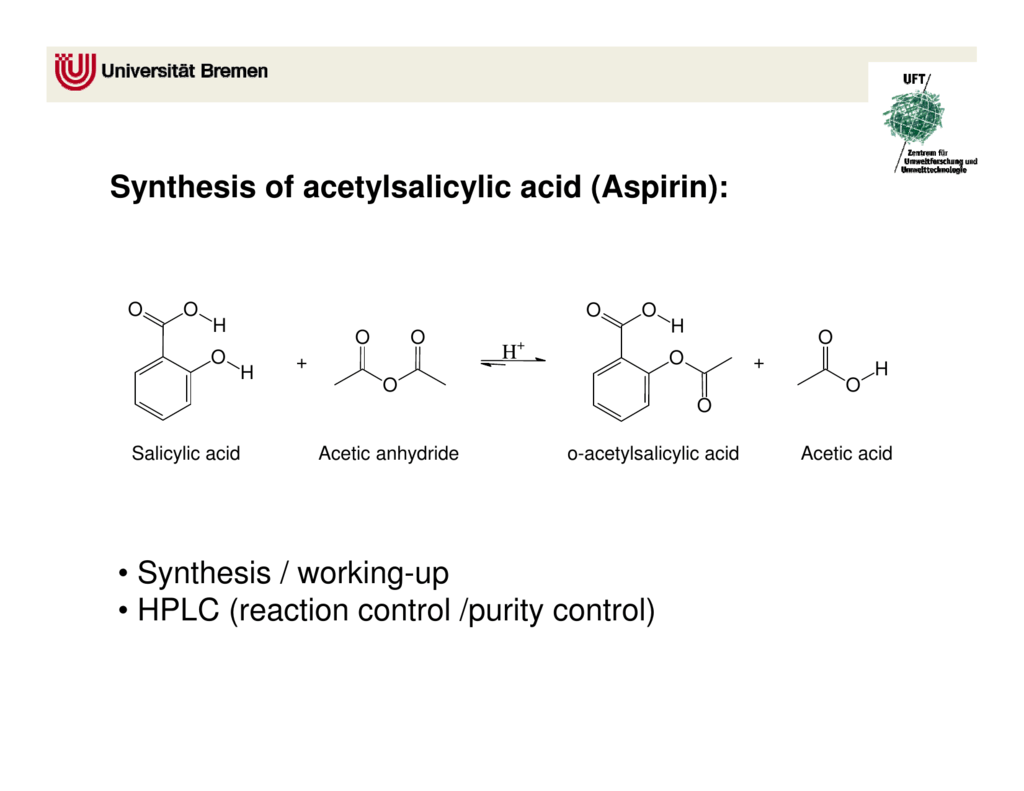 This picture illustrates synthesis of acetylsalicylic acid.
This picture illustrates synthesis of acetylsalicylic acid.
Synthesis of acetylsalicylic acid lab report
 This image shows Synthesis of acetylsalicylic acid lab report.
This image shows Synthesis of acetylsalicylic acid lab report.
Acetylsalicylic acid bond line
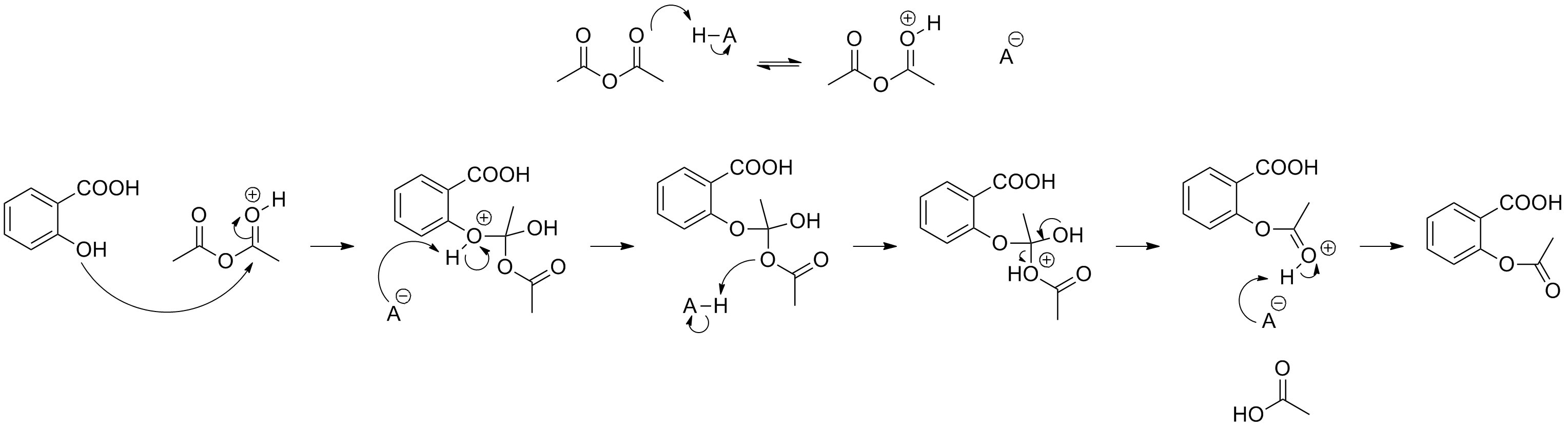 This image illustrates Acetylsalicylic acid bond line.
This image illustrates Acetylsalicylic acid bond line.
Synthesis of aspirin lab report discussion pdf
 This image representes Synthesis of aspirin lab report discussion pdf.
This image representes Synthesis of aspirin lab report discussion pdf.
Synthesis of aspirin balanced equation
 This picture illustrates Synthesis of aspirin balanced equation.
This picture illustrates Synthesis of aspirin balanced equation.
Preparation of aspirin project pdf
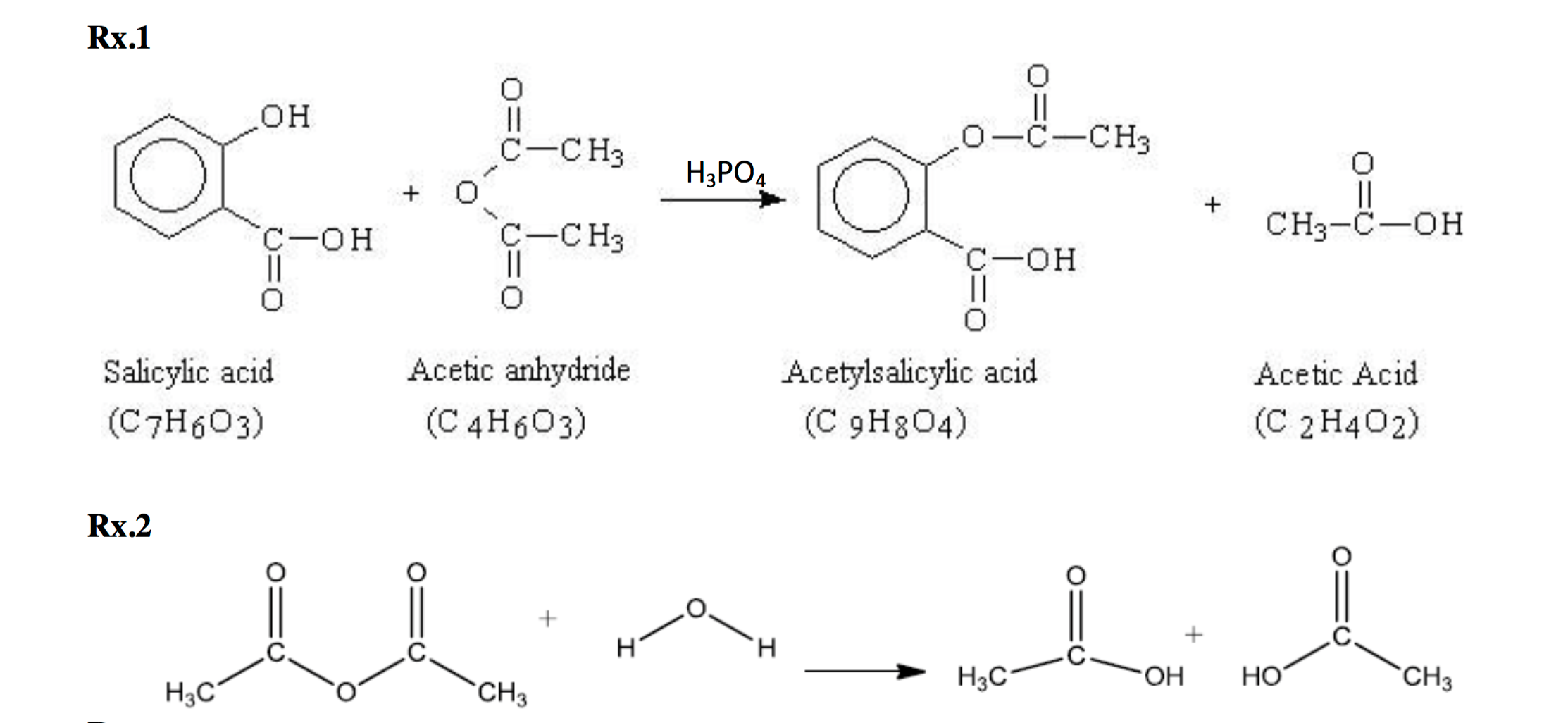 This picture shows Preparation of aspirin project pdf.
This picture shows Preparation of aspirin project pdf.
Synthesis of aspirin mechanism
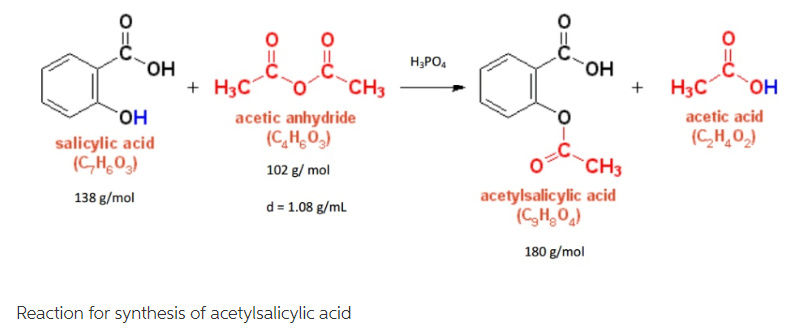 This picture illustrates Synthesis of aspirin mechanism.
This picture illustrates Synthesis of aspirin mechanism.
Industrial production of acetylsalicylic acid
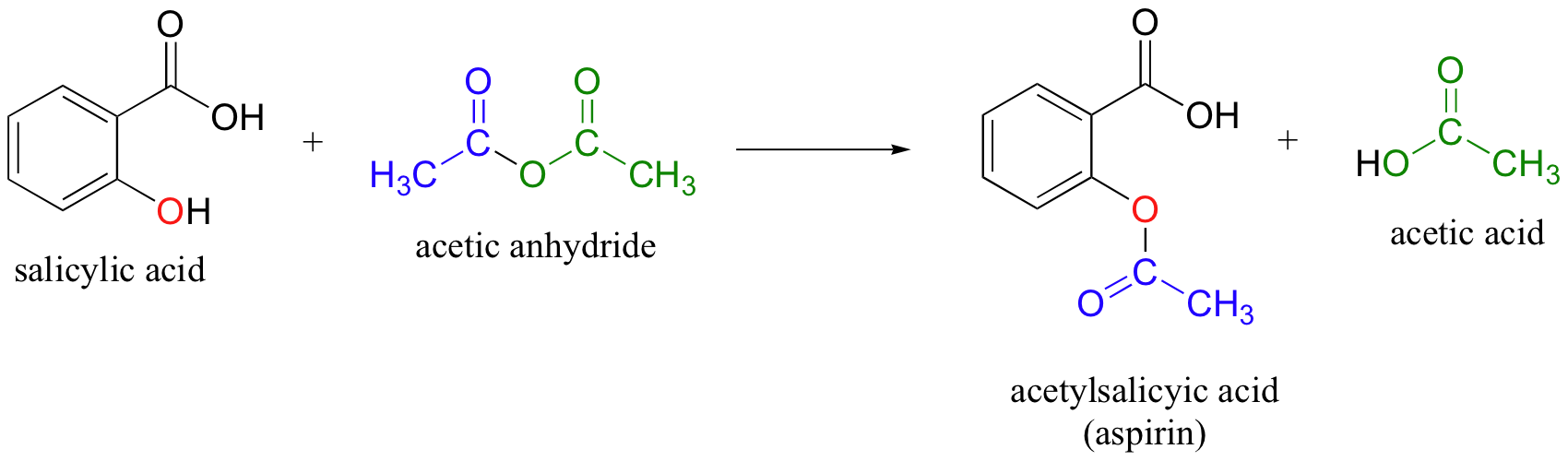 This picture shows Industrial production of acetylsalicylic acid.
This picture shows Industrial production of acetylsalicylic acid.
How to calculate the mass of acetyl salicylic acid?
Therefore moles acetylsalicylic acid (aspirin) = 0. 3623 mol To calculate the mass of aspirin that should be produced theoretically use the equation: m = mr * mol Mr of acetylsalicylic acid = 180 Therefore 180 * 0. 3623 = 65. 22g The above calculations show that theoretically there should be 65. 22g of aspirin produced.
How does salicylic acid react with aspirin?
This experiment was carried out to see how the hydroxyl group on the benzene ring in salicylic acid reacts with acetic anhydride to form an ester, and to make aspirin. Synthesis of Acetylsalicylic Acid occurs by protonation of carbonyl (C=O) group, and a nucleophilic attack of OH on the acetic anhydride.
How is acetic acid used in the synthesis of aspirin?
A small amount of a strong acid is used as a catalyst that speeds up the reaction. In this experiment, phosphoric acid will be used as the catalyst. The excess acetic acid will be quenched with the addition of water. The aspirin product is not very soluble in water so the aspirin product will
How is the synthesis of acetylsalicylic acid carried out?
Theory. This experiment was carried out to see how the hydroxyl group on the benzene ring in salicylic acid reacts with acetic anhydride to form an ester, and to make aspirin. Synthesis of Acetylsalicylic Acid occurs by protonation of carbonyl (C=O) group, and a nucleophilic attack of OH on the acetic anhydride.
Last Update: Oct 2021