Do you desperately look for 'how to write redox equations'? You can find all of the material on this webpage.
Write out a skeleton equivalence for the chemical reaction . The frame equation for the reaction on which this titration is based can Be written as follows. ...Assign oxidation Numbers to atoms connected both sides of the equation. ...Determine which atoms ar oxidized and which are reduced.
Table of contents
- How to write redox equations in 2021
- Redox reaction equations
- How to write half equations for redox reactions
- Balancing redox reactions calculator
- Balancing redox reactions - examples
- Redox half equations
- Net redox reaction
- 10 examples of redox reaction equations
How to write redox equations in 2021
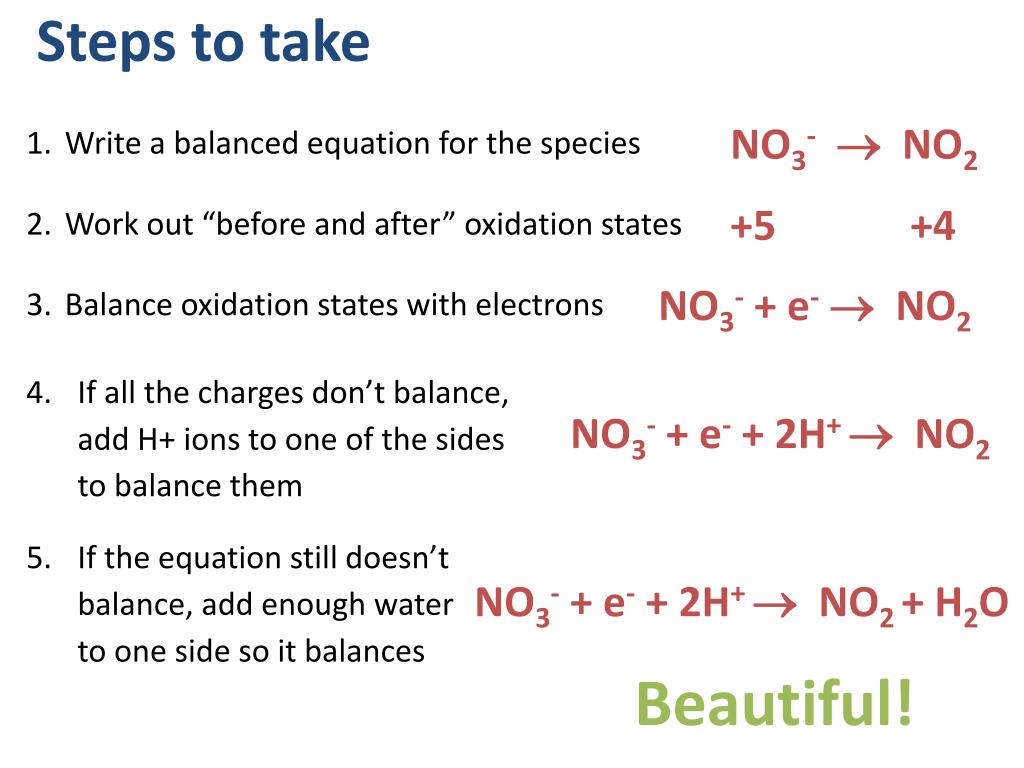 This picture representes how to write redox equations.
This picture representes how to write redox equations.
Redox reaction equations
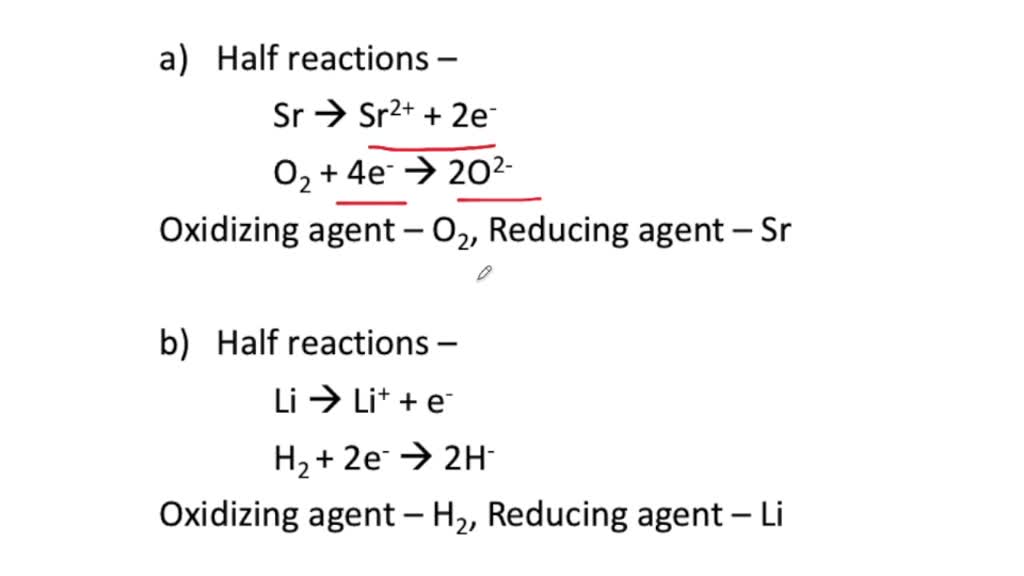 This image shows Redox reaction equations.
This image shows Redox reaction equations.
How to write half equations for redox reactions
 This picture representes How to write half equations for redox reactions.
This picture representes How to write half equations for redox reactions.
Balancing redox reactions calculator
 This picture shows Balancing redox reactions calculator.
This picture shows Balancing redox reactions calculator.
Balancing redox reactions - examples
 This picture demonstrates Balancing redox reactions - examples.
This picture demonstrates Balancing redox reactions - examples.
Redox half equations
 This image illustrates Redox half equations.
This image illustrates Redox half equations.
Net redox reaction
 This image representes Net redox reaction.
This image representes Net redox reaction.
10 examples of redox reaction equations
 This image illustrates 10 examples of redox reaction equations.
This image illustrates 10 examples of redox reaction equations.
What are the two halves of a redox reaction?
A redox reaction is made up of two half-reactions: (i) A reduction half-reaction in which one species, X, gains a electrons. X + ae - → X a-. (ii) An oxidation half-reaction in which one species, M, loses b electrons. M → M b+ + be -.
What are the guidelines for balancing redox equations?
Guidelines for Balancing Redox Equations: Determine the oxidation states of each species. Write each half reaction and for each: Balance atoms that change oxidation state. Balance the number of electrons transferred for each half reaction using the appropriate factor so that the electrons cancel.
How to write equations for redox reactions in chemistry?
This is easily resolved by adding two electrons to the left-hand side. The fully balanced half-reaction is: C l 2 + 2 e − → 2 C l −. Next the iron half-reaction is considered. Iron (II) ions are oxidized to iron (III) ions as shown: F e 2 + → F e 3 +.
How to write oxidation and reduction half reactions?
Write the oxidation and reduction half-reactions for the species that is reduced or oxidized. Multiply the half-reactions by the appropriate number so that they have equal numbers of electrons. Add the two equations to cancel out the electrons.
Last Update: Oct 2021
Leave a reply
Comments
Alphea
24.10.2021 08:48You will learn how these rules were derived.